to further advance major innovation in surgical single-handed suturing using the Switch®, supported by new financing
Abdominal suturing tests show that the Switch® facilitates the use of ‘small-bites’ technique for sustainable abdominal wall closure, preventing incisional hernia
- Study in The Lancet* by investigators from Erasmus MC Rotterdam showing ‘small bites’ should become the standard closure technique for midline incisions
- Single patient use Switch® device allows for higher precision ‘small-bites’ suturing of layered abdominal structures, twice as fast as the conventional technique.
- Laparotomies closed with classical big-bites technique develop a hernia in 5-35% and even up to 69%** in high risk patient groups.
- Cost savings of using the Switch® with small-bite approach could lead to up to $2 billion per year in the US alone.
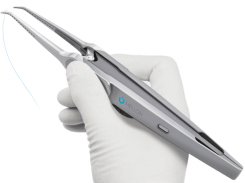
The Hague, The Netherlands, 15 June 2020 – Mellon Medical, a Dutch MedTech company focusing on the introduction of single handed surgical suturing devices, today announced the signing of an agreement with (medical) technology and systems designer Demcon of Enschede, The Netherlands, to further advance the Switch® towards market introduction. The Switch® is a (single patient use) precision surgical suturing device for abdominal wall closure, ideal for enabling the ‘small bites’ technique. This will reduce the risk of complications such as post-surgery incisional hernia, resulting in improved patient outcome and a substantial reduction of healthcare costs.
Along with this agreement Demcon will become a new shareholder in Mellon, contributing to the € 4 million in new development funds together with existing shareholders BGV (BioGeneration Ventures), OostNL, Brabantse Ontwikkelings Maatschappij (Brabant Development Company), David Pyott and Thuja Capital.
*Deerenberg et al, Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomized controlled trial, The Lancet July 16, 2015
**F. E. Muysoms et al, European Hernia Society guidelines on the closure of abdominal wall incisions, Hernia, 25 January 2015
Laparatomies: high risk and high health costs
Laparotomies involve large incisions through the abdominal wall to gain access into the abdominal cavity – procedures such as visceral, gynecological, aortic vascular, or urological surgery. In the US, 4-5 million laparotomies are done annually. The current practice is the big-bites technique using the classical suturing tools. Clinical studies have shown that big-bites techniques relate to a high occurrence of incisional hernia of up to 35% and even up to 69% in high risk patients, reducing the patients’ quality of life. This also has a major impact on healthcare costs. In US alone, more than 400.000 repairs are done annually, costing more than $4 billion. And still, repair of incisional hernia has a high failure rate of over 30%, even with the use of surgical mesh. Using the Switch® with small-bite technique would lead to cost savings of up to $2 billion per year in the US alone.
Lancet Study and preliminary abdominal suturing test in ErasmusMC leading in focus on abdominal small bite approach.
A large Dutch investigator funded double blind, multicentre, randomized controlled trial, at surgical and gynecological departments in ten hospitals in the Netherlands, published in The Lancet in 2015, clearly showed that the small bites suture technique is more effective than the traditional large bites technique for prevention of incisional hernia. Therefore, it should become the standard closure technique for midline incisions. Tests with the prototype were done in November 2019 at Erasmus MC in Rotterdam, the Netherlands, by two experienced surgeons. Their feedback was that the Switch’s straight needle for fascia is preferable to a curved needle and that it resulted in more regular suturing patterns. In addition, the surgeons experienced the design ergonomic and easy to use.
Prof. dr. Hans Jeekel, MD/PhD, Full Professor, Erasmus MC, and co-investigator and -author of The Lancet article, commenting on the opportunities of the newly developed suturing device for abdominal wall closing:
“The use of small bites significantly reduces the occurrence of incisional hernias. Therefore, it is essential that the small-bites technique would be used by any surgeon who closes the abdomen after surgery. And if that technique can be accelerated and facilitated with a device, that could be very important.”
Jan Benschop, CEO of Mellon Medical, commenting on this new direction of the company, said:
“By working with medical specialists, and the new collaboration with the renowned and highly skilled medical technology designer Demcon, being also one of our key new investors, and ergonomic experts in the development of the Switch®, Mellon will be able to reinvent suturing, bringing innovative suturing technology into the 21st century. Our mission is to improve medical procedures by providing the best possible tools. We believe our innovative platform suturing technology will improve patient outcome and reduce overall healthcare costs.”
Conventional suturing versus The Switch®
Classical suturing is a complex process and takes a long time to learn. Focus is on getting control over the needle. Surgeons using conventional suturing techniques need many coordinated motions using both hands in order to place a single suture. On average, 30% of the operation time is spent on suturing.
Mellon has managed to reinvent the technique of suturing. The Switch® can be operated by lightly pinching the double-action buttons with thumb and index finger of one hand. The other hand is free to present the tissue to be sutured. This technique greatly improves the precision and efficiency of the suturing process, as surgeons no longer need to switch the needle between instruments and focus on getting control over the needle.
In the Switch®, the needle is always secured in one of the two jaws. The predictable linear path followed by the straight needle causes less motion friction, resulting in a high-quality connection of the tissue layers.
FDA approval and CE mark
Mellon expects market introduction of the Switch® – which has been successfully tested by experienced surgeons in the Rotterdam Erasmus Medical Centre – in about 3 years, once final development and the FDA approval process have been completed. The CE mark process runs parallel to the FDA approval procedure, but is expected to be completed after market introduction.
The annual worldwide sutures and suturing devices market size is estimated over $3 billion by 2023.